At‑Home OTC COVID-19 Test
QuickVue At-Home OTC COVID-19 Test lets you get rapid results, in the privacy of your own home. Available over-the-counter, everything you need is in the package and taking the test is simple.
The test is authorized for home use with self-collected anterior nasal (nares) swab samples in individuals aged 2 and older. This test is also authorized for home use for individuals aged 2 through 14 with an adult performing the test. The test is intended to be used twice over two to three days, with at least 24 hours and no more than 36 hours between tests.
For In Vitro Diagnostic (IVD) Use.
For In Vitro Diagnostic (IVD) Use.
The test uses a gentle self-collected anterior nasal (nares) swab sample to determine a positive or negative COVID-19 result. The swab is swirled in a tube of reagent solution, then removed, before a test strip is inserted. After ten minutes, you can take the strip out of the tube and see your results.
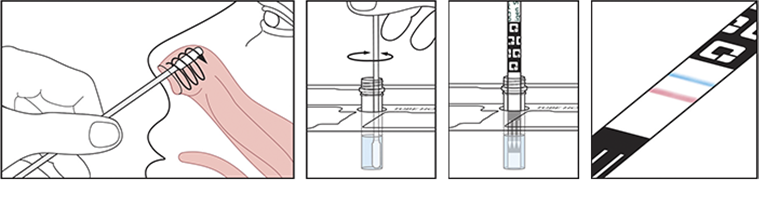
The test uses a gentle self-collected anterior nasal (nares) swab sample to determine a positive or negative COVID-19 result. The swab is swirled in a tube of reagent solution, then removed, before a test strip is inserted. After ten minutes, you can take the strip out of the tube and see your results.

At-Home OTC COVID-19 Test
QuidelOrtho is a California-based leading diagnostic manufacturer focused on improving the quality of healthcare throughout the globe. As a trusted innovator in the medical device industry for over 40 years, QuidelOrtho launched the QuickVue brand in 1986 with visually read rapid diagnostics focusing on women’s health and respiratory diseases. In 1999, QuickVue Influenza A+B was the first visually read rapid test cleared by the FDA for professional use.
Now, the QuickVue At-Home OTC COVID-19 Test utilizes trusted technology used for decades by healthcare professionals and makes simple, easy-to-use COVID-19 tests accessible for the good of our families, our communities and the world.

At-Home OTC COVID-19 Test
QuidelOrtho is a California-based leading diagnostic manufacturer focused on improving the quality of healthcare throughout the globe. As a trusted innovator in the medical device industry for over 40 years, QuidelOrtho launched the QuickVue brand in 1986 with visually read rapid diagnostics focusing on women’s health and respiratory diseases. In 1999, QuickVue Influenza A+B was the first visually read rapid test cleared by the FDA for professional use.
Now, the QuickVue At-Home OTC COVID-19 Test utilizes trusted technology used for decades by healthcare professionals and makes simple, easy-to-use COVID-19 tests accessible for the good of our families, our communities and the world.

The QuickVue At-Home OTC COVID-19 Test is a lateral flow immunoassay that allows for the rapid, qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 from individuals with or without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 36 hours between tests. This test is authorized for home use with self-collected (unobserved) direct anterior nasal (NS) swab specimens from individuals aged 14 years and older or with adult-collected anterior NS samples from individuals aged 2 years or older.